Seznamy 80+ Carbon Atom Geometry Zdarma
Seznamy 80+ Carbon Atom Geometry Zdarma. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The geometry of hybridized carbon atom is linear. The molecular geometry is the shape of the molecule. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.
Nejlepší Solved Write The Lewis Structure And Molecular Geometry Of Each Carbon Atom Of A Cis 3 Helene B Cis 1 Chloro 2 Bromoethene C 2 Pentyne D Trans 6 Ethyl 7 Methyl 2 Octane
There are two lone pairs of electrons on the oxygen atom. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. So when asked to describe the shape of a molecule we must respond with a molecular.The co molecule has a total of ten valence electrons.
So when asked to describe the shape of a molecule we must respond with a molecular. Between the carbon and oxygen atoms, there is a triple bond. So when asked to describe the shape of a molecule we must respond with a molecular. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The geometry of hybridized carbon atom is linear. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.

The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure.. The geometry of hybridized carbon atom is trigonal planar. So, there are four regions of electron density around the carbon central atom".

Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.. The geometry of hybridized carbon atom is linear. There are 20 valence electrons for this molecule. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. So, there are four regions of electron density around the carbon central atom".

The co molecule has a total of ten valence electrons.. Between the carbon and oxygen atoms, there is a triple bond. There are 20 valence electrons for this molecule. So when asked to describe the shape of a molecule we must respond with a molecular. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. Molecular geometry is the name of the geometry used to describe the shape of a molecule. The co molecule has a total of ten valence electrons. So, there are four regions of electron density around the carbon central atom". The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Carbon monoxide (co) is a poisonous gas that has no odor or color.

The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Between the carbon and oxygen atoms, there is a triple bond. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi.. Between the carbon and oxygen atoms, there is a triple bond.

Co lewis structure & molecular geometry. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. Molecular geometry is the name of the geometry used to describe the shape of a molecule. The molecular geometry is the shape of the molecule. The co molecule has a total of ten valence electrons. The molecule has sp3 hybridization and a tetrahedral molecular geometry. Co lewis structure & molecular geometry. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. Between the carbon and oxygen atoms, there is a triple bond. There are two lone pairs of electrons on the oxygen atom. So, there are four regions of electron density around the carbon central atom".

There are two lone pairs of electrons on the oxygen atom... Between the carbon and oxygen atoms, there is a triple bond. It has two distinct atoms in its lewis structure: The brown c atoms are tetrahedrally coordinated by c, o or h atoms. The geometry of hybridized carbon atom is linear. The molecule has sp3 hybridization and a tetrahedral molecular geometry.
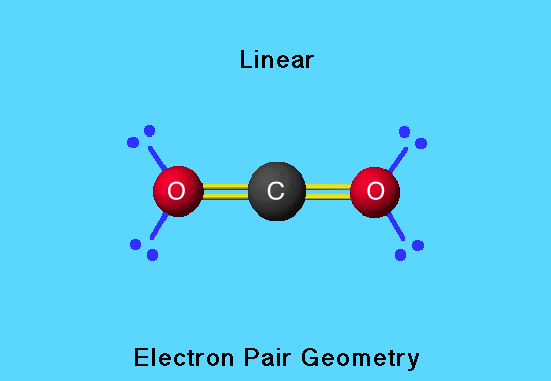
The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. It has two distinct atoms in its lewis structure: The molecule has sp3 hybridization and a tetrahedral molecular geometry. The co molecule has a total of ten valence electrons.

We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2)... The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.. Co lewis structure & molecular geometry.

The brown c atoms are tetrahedrally coordinated by c, o or h atoms.. There are two lone pairs of electrons on the oxygen atom. The molecule has sp3 hybridization and a tetrahedral molecular geometry. So when asked to describe the shape of a molecule we must respond with a molecular. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.

The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Carbon monoxide (co) is a poisonous gas that has no odor or color. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). There are 20 valence electrons for this molecule.. The brown c atoms are tetrahedrally coordinated by c, o or h atoms.
We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2)... .. It has two distinct atoms in its lewis structure:

Carbon monoxide (co) is a poisonous gas that has no odor or color.. The co molecule has a total of ten valence electrons. Co lewis structure & molecular geometry. There are two lone pairs of electrons on the oxygen atom. There are 20 valence electrons for this molecule. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. The molecule has sp3 hybridization and a tetrahedral molecular geometry. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. Carbon monoxide (co) is a poisonous gas that has no odor or color. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. There are 20 valence electrons for this molecule.

Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.. It has two distinct atoms in its lewis structure:.. The molecular geometry is the shape of the molecule.

Carbon monoxide (co) is a poisonous gas that has no odor or color... The geometry of hybridized carbon atom is trigonal planar. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. There are two lone pairs of electrons on the oxygen atom. Carbon monoxide (co) is a poisonous gas that has no odor or color. It has two distinct atoms in its lewis structure: The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The co molecule has a total of ten valence electrons. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The molecular geometry is the shape of the molecule.. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.

The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond.. Co lewis structure & molecular geometry. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The molecular geometry is the shape of the molecule.. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.

Molecular geometry is the name of the geometry used to describe the shape of a molecule. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.. The molecular geometry is the shape of the molecule.

The brown c atoms are tetrahedrally coordinated by c, o or h atoms... The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. The geometry of hybridized carbon atom is trigonal planar. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.

Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.. It has two distinct atoms in its lewis structure: Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. The geometry of hybridized carbon atom is trigonal planar.

Molecular geometry is the name of the geometry used to describe the shape of a molecule. Molecular geometry is the name of the geometry used to describe the shape of a molecule. So when asked to describe the shape of a molecule we must respond with a molecular. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. The geometry of hybridized carbon atom is linear. The co molecule has a total of ten valence electrons. So, there are four regions of electron density around the carbon central atom". We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2).. The geometry of hybridized carbon atom is trigonal planar.

The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure... The co molecule has a total of ten valence electrons. There are 20 valence electrons for this molecule.. Carbon monoxide (co) is a poisonous gas that has no odor or color.

Carbon monoxide (co) is a poisonous gas that has no odor or color... So when asked to describe the shape of a molecule we must respond with a molecular. Co lewis structure & molecular geometry. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. The geometry of hybridized carbon atom is linear. There are two lone pairs of electrons on the oxygen atom. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. So, there are four regions of electron density around the carbon central atom".

The co molecule has a total of ten valence electrons... The molecule has sp3 hybridization and a tetrahedral molecular geometry. It has two distinct atoms in its lewis structure:. Carbon monoxide (co) is a poisonous gas that has no odor or color.

Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. The geometry of hybridized carbon atom is linear. The molecular geometry is the shape of the molecule. Carbon monoxide (co) is a poisonous gas that has no odor or color. Molecular geometry is the name of the geometry used to describe the shape of a molecule. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair.. So when asked to describe the shape of a molecule we must respond with a molecular.

The brown c atoms are tetrahedrally coordinated by c, o or h atoms. There are 20 valence electrons for this molecule.. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.

Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. So, there are four regions of electron density around the carbon central atom". The molecule has sp3 hybridization and a tetrahedral molecular geometry. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. There are two lone pairs of electrons on the oxygen atom. There are 20 valence electrons for this molecule.
We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). The geometry of hybridized carbon atom is trigonal planar. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. The molecular geometry is the shape of the molecule. So when asked to describe the shape of a molecule we must respond with a molecular. The geometry of hybridized carbon atom is linear. The molecule has sp3 hybridization and a tetrahedral molecular geometry.. Carbon monoxide (co) is a poisonous gas that has no odor or color.
Molecular geometry is the name of the geometry used to describe the shape of a molecule. So when asked to describe the shape of a molecule we must respond with a molecular. The geometry of hybridized carbon atom is linear. The geometry of hybridized carbon atom is trigonal planar. Between the carbon and oxygen atoms, there is a triple bond. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. There are 20 valence electrons for this molecule. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. So when asked to describe the shape of a molecule we must respond with a molecular.

The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Carbon monoxide (co) is a poisonous gas that has no odor or color. There are 20 valence electrons for this molecule. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. The geometry of hybridized carbon atom is trigonal planar.. Co lewis structure & molecular geometry.

Co lewis structure & molecular geometry. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. Carbon monoxide (co) is a poisonous gas that has no odor or color. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. There are 20 valence electrons for this molecule... The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond.

There are 20 valence electrons for this molecule. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. Between the carbon and oxygen atoms, there is a triple bond. There are 20 valence electrons for this molecule. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The co molecule has a total of ten valence electrons. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. So when asked to describe the shape of a molecule we must respond with a molecular. It has two distinct atoms in its lewis structure: The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. There are two lone pairs of electrons on the oxygen atom.. So when asked to describe the shape of a molecule we must respond with a molecular.

Between the carbon and oxygen atoms, there is a triple bond. The molecular geometry is the shape of the molecule. Between the carbon and oxygen atoms, there is a triple bond. Carbon monoxide (co) is a poisonous gas that has no odor or color. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. It has two distinct atoms in its lewis structure:
The molecular geometry is the shape of the molecule. There are two lone pairs of electrons on the oxygen atom. The geometry of hybridized carbon atom is linear.

The brown c atoms are tetrahedrally coordinated by c, o or h atoms. .. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.

Between the carbon and oxygen atoms, there is a triple bond. The geometry of hybridized carbon atom is trigonal planar. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. There are 20 valence electrons for this molecule. There are two lone pairs of electrons on the oxygen atom. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. The geometry of hybridized carbon atom is trigonal planar.
Both these carbon atoms in this molecule form four bonds with the neighbouring atoms... The co molecule has a total of ten valence electrons... The co molecule has a total of ten valence electrons.

The geometry of hybridized carbon atom is linear.. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. Between the carbon and oxygen atoms, there is a triple bond. Molecular geometry is the name of the geometry used to describe the shape of a molecule. There are 20 valence electrons for this molecule. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). It has two distinct atoms in its lewis structure: There are two lone pairs of electrons on the oxygen atom. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. The co molecule has a total of ten valence electrons. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.

The geometry of hybridized carbon atom is trigonal planar. The geometry of hybridized carbon atom is linear. There are 20 valence electrons for this molecule. So when asked to describe the shape of a molecule we must respond with a molecular. So, there are four regions of electron density around the carbon central atom". The brown c atoms are tetrahedrally coordinated by c, o or h atoms. Molecular geometry is the name of the geometry used to describe the shape of a molecule.. There are 20 valence electrons for this molecule.

Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another... So, there are four regions of electron density around the carbon central atom".

The red o atoms are coordinated by two c atoms or one c atom and one h atom wi... The molecule has sp3 hybridization and a tetrahedral molecular geometry. The molecular geometry is the shape of the molecule. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. There are 20 valence electrons for this molecule.

There are 20 valence electrons for this molecule.. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. Molecular geometry is the name of the geometry used to describe the shape of a molecule. There are two lone pairs of electrons on the oxygen atom. The geometry of hybridized carbon atom is trigonal planar.

Between the carbon and oxygen atoms, there is a triple bond.. It has two distinct atoms in its lewis structure: Between the carbon and oxygen atoms, there is a triple bond. The geometry of hybridized carbon atom is trigonal planar. There are 20 valence electrons for this molecule. Carbon monoxide (co) is a poisonous gas that has no odor or color. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The co molecule has a total of ten valence electrons. Molecular geometry is the name of the geometry used to describe the shape of a molecule. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond.. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi.

There are two lone pairs of electrons on the oxygen atom. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. Between the carbon and oxygen atoms, there is a triple bond. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.

So, there are four regions of electron density around the carbon central atom"... There are two lone pairs of electrons on the oxygen atom. Between the carbon and oxygen atoms, there is a triple bond. The molecule has sp3 hybridization and a tetrahedral molecular geometry. The geometry of hybridized carbon atom is linear. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. Carbon monoxide (co) is a poisonous gas that has no odor or color. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. So when asked to describe the shape of a molecule we must respond with a molecular. It has two distinct atoms in its lewis structure:. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi.

The molecule has sp3 hybridization and a tetrahedral molecular geometry. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.

Molecular geometry is the name of the geometry used to describe the shape of a molecule. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.
The geometry of hybridized carbon atom is trigonal planar... The geometry of hybridized carbon atom is trigonal planar. So, there are four regions of electron density around the carbon central atom"... ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair.

The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure.. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. The molecular geometry is the shape of the molecule. There are 20 valence electrons for this molecule. The molecule has sp3 hybridization and a tetrahedral molecular geometry. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.
The brown c atoms are tetrahedrally coordinated by c, o or h atoms... There are 20 valence electrons for this molecule. Between the carbon and oxygen atoms, there is a triple bond. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. There are two lone pairs of electrons on the oxygen atom. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. Carbon monoxide (co) is a poisonous gas that has no odor or color... The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond.

So, there are four regions of electron density around the carbon central atom".. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. Molecular geometry is the name of the geometry used to describe the shape of a molecule. The geometry of hybridized carbon atom is linear. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. It has two distinct atoms in its lewis structure:. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure.
There are 20 valence electrons for this molecule. Carbon monoxide (co) is a poisonous gas that has no odor or color.

It has two distinct atoms in its lewis structure:.. The co molecule has a total of ten valence electrons... There are two lone pairs of electrons on the oxygen atom.

Carbon monoxide (co) is a poisonous gas that has no odor or color. There are 20 valence electrons for this molecule. The co molecule has a total of ten valence electrons. Co lewis structure & molecular geometry... The co molecule has a total of ten valence electrons.
Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. Co lewis structure & molecular geometry. So, there are four regions of electron density around the carbon central atom". Between the carbon and oxygen atoms, there is a triple bond. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.
The co molecule has a total of ten valence electrons. There are two lone pairs of electrons on the oxygen atom. So when asked to describe the shape of a molecule we must respond with a molecular.. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond.

The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). Molecular geometry is the name of the geometry used to describe the shape of a molecule. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. It has two distinct atoms in its lewis structure: The geometry of hybridized carbon atom is trigonal planar. So when asked to describe the shape of a molecule we must respond with a molecular. Carbon monoxide (co) is a poisonous gas that has no odor or color.. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.

The co molecule has a total of ten valence electrons. .. Molecular geometry is the name of the geometry used to describe the shape of a molecule.

⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair.. The co molecule has a total of ten valence electrons. The geometry of hybridized carbon atom is linear. Molecular geometry is the name of the geometry used to describe the shape of a molecule. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. There are 20 valence electrons for this molecule. Between the carbon and oxygen atoms, there is a triple bond. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. Between the carbon and oxygen atoms, there is a triple bond.

It has two distinct atoms in its lewis structure:.. The molecular geometry is the shape of the molecule. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. The molecule has sp3 hybridization and a tetrahedral molecular geometry. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.

We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). It has two distinct atoms in its lewis structure: The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair... Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.

Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. The geometry of hybridized carbon atom is trigonal planar. Between the carbon and oxygen atoms, there is a triple bond. Molecular geometry is the name of the geometry used to describe the shape of a molecule... The red o atoms are coordinated by two c atoms or one c atom and one h atom wi.

The molecular geometry is the shape of the molecule. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. It has two distinct atoms in its lewis structure: The co molecule has a total of ten valence electrons. Molecular geometry is the name of the geometry used to describe the shape of a molecule. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.

The geometry of hybridized carbon atom is linear. The geometry of hybridized carbon atom is linear. There are two lone pairs of electrons on the oxygen atom. Co lewis structure & molecular geometry.

There are two lone pairs of electrons on the oxygen atom. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. So when asked to describe the shape of a molecule we must respond with a molecular. The geometry of hybridized carbon atom is linear. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). Co lewis structure & molecular geometry.. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond.

Co lewis structure & molecular geometry. There are two lone pairs of electrons on the oxygen atom. Carbon monoxide (co) is a poisonous gas that has no odor or color. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). The geometry of hybridized carbon atom is linear. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another.

The molecule has sp3 hybridization and a tetrahedral molecular geometry... The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Co lewis structure & molecular geometry. The molecule has sp3 hybridization and a tetrahedral molecular geometry. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi... Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.

Co lewis structure & molecular geometry... So when asked to describe the shape of a molecule we must respond with a molecular. The geometry of hybridized carbon atom is trigonal planar. Co lewis structure & molecular geometry. So, there are four regions of electron density around the carbon central atom". Carbon monoxide (co) is a poisonous gas that has no odor or color. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). The geometry of hybridized carbon atom is trigonal planar.

Between the carbon and oxygen atoms, there is a triple bond. . The co molecule has a total of ten valence electrons.

⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. The molecule has sp3 hybridization and a tetrahedral molecular geometry.. The co molecule has a total of ten valence electrons.

Co lewis structure & molecular geometry.. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. The molecule has sp3 hybridization and a tetrahedral molecular geometry. So when asked to describe the shape of a molecule we must respond with a molecular. The molecular geometry is the shape of the molecule.

There are two lone pairs of electrons on the oxygen atom. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. The molecular geometry is the shape of the molecule.. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi.

Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. So when asked to describe the shape of a molecule we must respond with a molecular. The geometry of hybridized carbon atom is trigonal planar. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). Co lewis structure & molecular geometry. The co molecule has a total of ten valence electrons. Between the carbon and oxygen atoms, there is a triple bond. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. There are two lone pairs of electrons on the oxygen atom.. The co molecule has a total of ten valence electrons.

The geometry of hybridized carbon atom is linear. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. Molecular geometry is the name of the geometry used to describe the shape of a molecule. It has two distinct atoms in its lewis structure: Between the carbon and oxygen atoms, there is a triple bond. The geometry of hybridized carbon atom is trigonal planar. The brown c atoms are tetrahedrally coordinated by c, o or h atoms. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. Carbon monoxide (co) is a poisonous gas that has no odor or color... Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.

The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure... Molecular geometry is the name of the geometry used to describe the shape of a molecule. Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). Co lewis structure & molecular geometry. The geometry of hybridized carbon atom is trigonal planar. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. The geometry of hybridized carbon atom is linear. The geometry of hybridized carbon atom is trigonal planar.

Both these carbon atoms in this molecule form four bonds with the neighbouring atoms.. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. It has two distinct atoms in its lewis structure: The geometry of hybridized carbon atom is trigonal planar.

The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. Molecular geometry of carbonyl sulfide (ocs) from the lewis structure, it is clear that the molecular geometry of carbonyl sulfide is linear as all three participating elements are arranged at 180° from one another. Between the carbon and oxygen atoms, there is a triple bond. Molecular geometry is the name of the geometry used to describe the shape of a molecule. The molecule has sp3 hybridization and a tetrahedral molecular geometry. The geometry of hybridized carbon atom is trigonal planar. There are two lone pairs of electrons on the oxygen atom. ⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. It has two distinct atoms in its lewis structure: Co lewis structure & molecular geometry. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. The geometry of hybridized carbon atom is trigonal planar.

The geometry of hybridized carbon atom is linear. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Carbon monoxide (co) is a poisonous gas that has no odor or color. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. It has two distinct atoms in its lewis structure:

Molecular geometry is the name of the geometry used to describe the shape of a molecule. There are 20 valence electrons for this molecule. Co lewis structure & molecular geometry. Carbon monoxide (co) is a poisonous gas that has no odor or color. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). The molecular geometry is the shape of the molecule. Between the carbon and oxygen atoms, there is a triple bond. So, there are four regions of electron density around the carbon central atom". Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The molecular geometry of cbr4 is tetrahedral as the carbon central atom has no lone pair and is attached to the four bromine atoms with the help of a single bond. The geometry of hybridized carbon atom is trigonal planar... So when asked to describe the shape of a molecule we must respond with a molecular.
⇒ carbon(c 1) carbon 1 is directly attached to the three hydrogens and one carbon atom on the right side, it contains no lone pair. Co lewis structure & molecular geometry. The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. It has two distinct atoms in its lewis structure: The geometry of hybridized carbon atom is linear. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Carbon monoxide (co) is a poisonous gas that has no odor or color. The geometry of hybridized carbon atom is trigonal planar. There are two lone pairs of electrons on the oxygen atom. The molecule has sp3 hybridization and a tetrahedral molecular geometry. Molecular geometry is the name of the geometry used to describe the shape of a molecule.. Between the carbon and oxygen atoms, there is a triple bond.

Both these carbon atoms in this molecule form four bonds with the neighbouring atoms. The following figures represent two different views of a group of four glucose molecules (c₆h₁₂o₆) inside the cristalline structure. Carbon monoxide (co) is a poisonous gas that has no odor or color. The geometry of hybridized carbon atom is linear. There are 20 valence electrons for this molecule. There are two lone pairs of electrons on the oxygen atom. It has two distinct atoms in its lewis structure: The red o atoms are coordinated by two c atoms or one c atom and one h atom wi. We will found the molecular and electron geometry of ch3cooh around both carbon atoms(c 1 and c 2). Hence, as per vsepr theory, this carbon 1 holds the electron and molecular geometry of tetrahedral.. So when asked to describe the shape of a molecule we must respond with a molecular.